
Instructions for use
KaVoLUX 540 LED U / T

Distributed by:
KaVo Dental GmbH
Bismarckring 39
D-88400 Biberach
Phone +49 (0) 7351 56-0
Fax +49 (0) 7351 56-1488
Manufacturer:
Kaltenbach & Voigt GmbH
Bismarckring 39
D-88400 Biberach
www.kavo.com

Table of contents
1 User instructions ................................................................................................................ 6
1.1 User guide .................................................................................................................... 6
1.1.1 Abbreviations ..................................................................................................... 6
1.1.2 Symbols ............................................................................................................ 6
1.1.3 Target group ..................................................................................................... 6
1.2 Service ......................................................................................................................... 7
1.3 Terms and conditions of warranty.................................................................................... 7
1.4 Transportation and storage............................................................................................. 7
1.4.1 Currently valid packaging regulations.................................................................... 7
1.4.2 Damage in transit ............................................................................................... 8
1.4.3 Information on the packaging: Storage and transportation...................................... 9
2 Safety..................................................................................................................................10
2.1 Description of safety instructions..................................................................................... 10
2.1.1 Warning symbol ................................................................................................. 10
2.1.2 Structure ........................................................................................................... 10
2.1.3 Description of hazardous steps............................................................................. 10
2.2 Safety instructions ......................................................................................................... 10
2.3 Disposal........................................................................................................................ 12
2.4 Information on electromagnetic compatibility.................................................................... 12
2.5 Disposal of electronic and electrical devices ...................................................................... 13
3 Product description ............................................................................................................14
3.1 Purpose – Proper use ..................................................................................................... 14
3.2 Operating light KaVoLUX 540 LED U ................................................................................ 16
3.3 Operating light KaVoLUX 540 LED T................................................................................. 17
3.4 Dimensions and swing range........................................................................................... 17
3.5 Rating plates................................................................................................................. 19
3.6 Technical data and requirements..................................................................................... 20
3.7 Overview of use............................................................................................................. 21
3.8 Accessories ................................................................................................................... 22
4 Assembly ............................................................................................................................23
4.1 Packaging ..................................................................................................................... 23
4.1.1 Transportation and storage.................................................................................. 23
4.1.2 Packed items ..................................................................................................... 23
4.1.3 Removal from the packaging................................................................................ 25
4.2 Requirements for mounting ........................................................................................... 27
4.2.1 Mounting the backstop to the swivel arm .............................................................. 27
4.2.2 Requirements for mounting the device.................................................................. 27
4.2.3 Requirements for ceiling mounting ....................................................................... 28
4.3 Assembly of the KaVoLUX 540 LED U............................................................................... 28
4.3.1 ESTETICA E50 / E50 Life ..................................................................................... 28
4.3.2 ESTETICA E70 / ESTETICA E80 ............................................................................ 32
4.3.3 ESTETICA E70/E80 Vision.................................................................................... 37
4.3.4 Activate KaVoLUX 540 LED U in service mode........................................................ 41
4.4 Assembly of the KaVoLUX 540 LED T ............................................................................... 45
4.4.1 ESTETICA E30.................................................................................................... 45
Instructions for use KaVoLUX 540 LED U / T
Table of contents
3 / 110

4.4.2 Primus 1058....................................................................................................... 49
4.4.3 Primus 1058 Life................................................................................................. 54
4.4.4 ESTETICA Standard 1063 / Comfort 1065 / Sensus 1066........................................ 58
4.4.5 Globus 1078 S, Status 1080 TM/C........................................................................ 61
4.5 Ceiling assembly of the KaVoLUX 540 LED T..................................................................... 64
4.6 Centro-mounting of the KaVoLUX 540 LED ....................................................................... 72
5 Mechanical settings ............................................................................................................74
5.1 Adjusting the swinging arm brake.................................................................................... 74
5.2 Set the spring arm brake................................................................................................ 74
5.3 Setting the light head brakes .......................................................................................... 75
5.3.1 Setting the rotary motion und 3D joint movement.................................................. 75
5.3.2 Adjusting the light head brake.............................................................................. 76
6 Operation............................................................................................................................78
6.1 Operation of the treatment light KaVoLUX 540 LED U ........................................................ 79
6.1.1 Turning the operating light On and Off .................................................................. 79
6.1.2 Setting the brightness ......................................................................................... 80
6.1.3 Changing between COMPOsave mode res. dimmed light and normal light................. 80
6.1.4 Switch on COMPOsave mode ............................................................................... 81
6.1.5 Setting the brightness of the dimmer (COMPOsave mode or normal light) ............... 82
6.1.6 Set colour temperature ....................................................................................... 82
6.1.7 Turning the laser mode On and Off....................................................................... 82
6.1.8 Operation of the 3D joint ..................................................................................... 84
6.2 Operation of the treatment light KaVoLUX 540 LED T ........................................................ 84
6.2.1 Turning the operating light On and Off .................................................................. 85
6.2.2 Setting the brightness ......................................................................................... 85
6.2.3 Changing between COMPOsave mode res. dimmed light and normal light................. 86
6.2.4 Switch on COMPOsave mode ............................................................................... 86
6.2.5 Setting the brightness of the dimmer.................................................................... 87
6.2.6 Set colour temperature ....................................................................................... 87
6.2.7 Turning the laser mode On and Off....................................................................... 87
6.2.8 Operation of the 3D joint ..................................................................................... 89
7 Rehabilitation methods according to DIN EN ISO 17664...................................................90
7.1 General care instructions ................................................................................................ 90
7.2 Cleaning and disinfection of light, glass cover and mirror ................................................... 90
7.3 Cleaning and disinfecting the handles............................................................................... 91
7.4 Sterilisation................................................................................................................... 91
7.4.1 Sterilising the handles ......................................................................................... 91
8 Safety check - Test instructions .........................................................................................92
8.1 Mounting the device....................................................................................................... 92
8.2 Ceiling mounting............................................................................................................ 92
8.2.1 Introduction ....................................................................................................... 92
8.2.2 Instructions for the safety check........................................................................... 95
8.2.3 Test protocol for the safety check .........................................................................103
9 Eliminating disturbances....................................................................................................104
10Specifications on the electromagnetic compatibility in accordance with EN 60601-1-2...105
10.1Guidelines and manufacturer's declaration - electromagnetic emission ................................105
Instructions for use KaVoLUX 540 LED U / T
Table of contents
4 / 110

10.2Guidelines and manufacturer's statement - Electromagnetic immunity ................................105
10.3Guidelines and manufacturer's statement - Electromagnetic immunity ................................106
10.4Recommended protective clearances between portable and mobile HF telecommunication
devices and the KaVoLUX 540 LED ..................................................................................108
Instructions for use KaVoLUX 540 LED U / T
Table of contents
5 / 110

Instructions for use KaVoLUX 540 LED U / T
1 User instructions | 1.1 User guide
6 / 110
1 User instructions
1.1 User guide
Requirement
Read these instructions before the initial startup to prevent misuse and dam-
age.
1.1.1 Abbreviations
Ab-
brevi-
ation
Explanation
IFU Instructions for use
CI Care instructions
KA Short instructions for use
AI Assembly instructions
TI Technician's instructions
IEC International Electrotechnical Commission
RI Repair instructions
RK Retrofitting kit
AS Assembly kit
CK Conversion kit
EP Enclosed parts
EMC Electromagnetic compatibility
PI Processing instructions
1.1.2 Symbols
See the Safety/Warning Symbols section
Important information for users and technicians
CE mark according to Medical Devices Directive EC 93/42
Action required
eLabeling ID
1.1.3 Target group
This document is for dental professionals, dental office staff and service staff.

Instructions for use KaVoLUX 540 LED U / T
1 User instructions | 1.2 Service
1.2 Service
KaVo Customer Service:
+49 (0) 7351 56-1000
service.einrichtungen@kavokerr.com or service.treatmentunits@kavokerr.com
Please refer to the serial number of the product in all inquiries!
For further information, please visit: www.kavo.com
1.3 Terms and conditions of warranty
KaVo provides the final customer with a warranty that the product cited in the
handover certificate will function properly and guarantees zero defects in the
material or processing for a period of 12 months from data of purchase, subject
to the following conditions:
Upon justified complaints of flaws or a short delivery, KaVo will make good its
warranty by replacing the product free of cost or repairing it according to the
customer's wishes. Other claims of any nature whatsoever, in particular with
respect to compensation, are excluded. In the event of default and gross negli-
gence or intent, this shall only apply in the absence of mandatory legal regula-
tions to the contrary.
KaVo cannot be held liable for defects and their consequences due to natural
wear, improper cleaning or servicing, non-compliance with operating, servicing
or connection instructions, calcification or corrosion, contaminated air or water
supplies or chemical or electrical factors deemed abnormal or impermissible in
accordance with factory specifications.
The warranty does not usually cover bulbs, glassware, rubber parts and the col-
ourfastness of plastics.
Defects or their consequences that can be attributed to interventions on or
changes made to the product by the customer or a third party are excluded
from the warranty.
Claims from this warranty can only be asserted when the transfer form (copy)
belonging to the product has been sent to KaVo, and the original can be
presented by the operator or user.
1.4 Transportation and storage
1.4.1 Currently valid packaging regulations
Note
Only valid for the Federal Republic of Germany.
Dispose of and recycle the sales packaging appropriately in accordance with
current packaging regulations, employing waste management or recycling com-
panies. Comply with the comprehensive return system. KaVo has had its sales
packaging licensed for this purpose. Please comply with the regional public
waste-disposal system.
7 / 110

Instructions for use KaVoLUX 540 LED U / T
1 User instructions | 1.4 Transportation and storage
8 / 110
1.4.2 Damage in transit
In Germany
If the packaging is visibly damaged on delivery, please proceed as follows:
1. The recipient of the package must record the loss or damage on the delivery
receipt. The recipient and the representative of the shipping company must
sign this delivery receipt.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use the product.
4. Report the damage to the shipping company.
5. Report the damage to KaVo.
6. Consult with KaVo first, before returning a damaged product.
7. Send the signed delivery receipt to KaVo.
If the product is damaged but there was no discernable damage to the pack-
aging on delivery, proceed as follows:
1. Report the damage to the shipping company immediately and no later than
7 days after delivery.
2. Report the damage to KaVo.
3. Leave the product and packaging in the condition in which you received it.
4. Do not use a damaged product.
Note
Failure on the part of the recipient to comply with any of the above-men-
tioned obligations will mean that the damage will be considered to have
arisen following delivery (in accordance with the General German Freight For-
warders' Terms and Conditions, Art. 28).
Outside Germany
Note
KaVo shall not be held liable for damage arising from transportation.
The shipment must be checked on arrival.
If the packaging is visibly damaged on delivery, please proceed as follows:
1. The recipient of the package must record the loss or damage on the delivery
receipt. The recipient and the representative of the shipping company must
sign this delivery receipt.
Without this evidence, the recipient will not be able to assert a claim for
damages against the shipping company.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use the product.
If the product is damaged but there was no discernable damage to the pack-
aging on delivery, proceed as follows:
1. Report any damage to the shipping company immediately and no later than
7 days after delivery.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use a damaged product.
Note
If the recipient fails to comply with any of the above-mentioned obligations,
the damage will be considered to have arisen following delivery
(in accordance with CMR law, Chapter 5, Art. 30).

Instructions for use KaVoLUX 540 LED U / T
1 User instructions | 1.4 Transportation and storage
1.4.3 Information on the packaging: Storage and
transportation
Note
Please keep the packaging in case you need to return the product for servi-
cing or repair.
The symbols printed on the outside are for transportation and storage, and
have the following meaning:
Transport upright with the arrows pointing upwards!
Fragile - protect against impact!
Protect from moisture!
Permissible stacking load
Temperature range
Humidity
Air pressure
9 / 110

Instructions for use KaVoLUX 540 LED U / T
2 Safety | 2.1 Description of safety instructions
10 / 110
2 Safety
2.1 Description of safety instructions
2.1.1 Warning symbol
Warning symbol
2.1.2 Structure
DANGER
The introduction describes the type and source of the hazard.
This section describes potential consequences of non-compliance.
▶The optional step includes necessary measures for hazard prevention.
2.1.3 Description of hazardous steps
The warning and safety notes in this document must be observed to prevent
personal injury and material damage. The warning notes are designated as
shown below:
DANGER
In cases which – if not prevented – directly lead to death or severe in-
jury.
WARNING
In cases which – if not prevented – can lead to death or severe injury.
CAUTION
In cases which – if not prevented – could lead to minor or moderate
injury.
NOTICE
In cases which – if not prevented – could lead to material damage.
2.2 Safety instructions
DANGER
Explosion hazard.
Risk of fatal injury.
▶Do not use KaVo product in areas subject an explosion hazard.

Instructions for use KaVoLUX 540 LED U / T
2 Safety | 2.2 Safety instructions
WARNING
Unintentional activation of the KaVo KEY Laser III and KEY Laser 3+.
Simultaneous application of the operating light KaVoLUX 540 LED and the
KaVo KEY Laser III or KEY Laser 3+ can lead to the unintentional activation of
the KaVo KEY Laser III and KEY Laser 3+.
▶When using the KaVo KEY Laser III or the KEY Laser 3+, switch the operat-
ing light to laser mode.
▶Or switch off the operating light, do not use the KaVo KEY Laser III or
KEY Laser 3+ and the KaVoLUX 540 LED operating light simultaneously.
CAUTION
Inadvertent activation of the sensor of the KaVoLUX 540 LED operat-
ing light by laser or third-party light.
The use of a laser or third-party light can lead to malfunction or trigger the
sensor of the KaVoLUX 540 LED and switch the operating light on or off inad-
vertently.
▶Have the sensor disabled by an authorised engineer.
CAUTION
Stroboscopic effect of the rotating instrument.
A stroboscopic effect could arise in instruments rotating at a certain speed dur-
ing application of the KaVoLUX 540 LED. This is an optical illusion that makes
the handpiece appear to be standing still or rotating extremely slowly.
Risk of injury.
▶If the stroboscopic effect is evident, change the speed slightly and continue
working as usual.
CAUTION
Premature hardening of composite fillings.
A light intensity that is too high can have a negative impact on the durability of
the treatment.
▶Select the appropriate dimming level according to the processing time.
CAUTION
Faulty measurement in connection with KaVo DIAGNOdent.
Simultaneous application of the operating light KaVoLUX 540 LED and the
KaVo DIAGNOdent can lead to faulty measurements.
▶Switch the operating light to laser mode when using the
KaVo DIAGNOdent.
▶Or switch off the operating light, do not use KaVo DIAGNOdent and operat-
ing light KaVoLUX 540 LED simultaneously.
CAUTION
Damage from unsuitable accessories.
The use of accessories, other components, and cables other than those spe-
cified (with the exception of components and cables sold by KaVo as spare
parts for internal components) can increase emissions or reduce the electro-
magnetic immunity of the product.
▶Use accessories recommended by KaVo only!
Note
Switch off the light during treatment intervals to increase the service life of
the LED light.
11 / 110

Instructions for use KaVoLUX 540 LED U / T
2 Safety | 2.3 Disposal
12 / 110
Compliance with the national statutory regulations is obligatory during the use
of the device, in particular in as far as it concerns:
▪ the applicable health and safety regulations
▪ the applicable accident prevention regulations
The user is responsible for:
▪ exclusive use of equipment that is operating correctly
▪ protecting him- or herself, the patient and third parties from danger
▪ preventing any contamination by means of the device
The following regulations must be observed for the ceiling assembly of the
KaVoLUX 540 LED T:
▪ Guidelines of the regulatory and building authorities
▪ Only use fastening elements (heavy-duty dowels) that are approved by the
building authorities
▪ The electrical installation may only be performed by a certified electrician
▪ The electrical installation must comply with the requirements and guidelines
set down in VDE 0100-710
The following regulations must be observed when the KaVoLUX 540 LED T is
mounted to a treatment center:
▪ The electrical installation must comply with the requirements and guidelines
set down in VDE 0100-710
The safety checks must be done at 24-month intervals.
See also:
28 Safety check - Test instructions, Page 92
2.3 Disposal
Note
Any waste which is generated must be recycled or disposed of in strict com-
pliance with all applicable national regulations in a manner which is safe both
for people and the environment.
If you have any questions regarding proper disposal of the KaVo product,
please contact the KaVo branch.
2.4 Information on electromagnetic compatibility
Note
Based on IEC 60601-1-2 (DIN EN 60601-1-2) concerning the electromag-
netic compatibility of electrical medical devices, we must draw your attention
to the following points:
• Electrical medical devices are subject to special precautionary measures re-
garding electromagnetic compatibility and must be operated in accordance
with the KaVo installation instructions.
• High-frequency communications devices can affect the operation of elec-
trical medical devices.
Further details of the technical EMC description are available on request.

Instructions for use KaVoLUX 540 LED U / T
2 Safety | 2.5 Disposal of electronic and electrical devices
See also:
210 Information about electromagnetic compatibility, Page 105
Note
KaVo cannot guarantee the compliance of accessories, cables, and other
components not supplied by KaVo with the EMC requirements of IEC
60601-1-2 (DIN EN 60601-1-2).
2.5 Disposal of electronic and electrical devices
Note
According to EC directive 2012/19 concerning waste electrical and electronic
equipment, this product is subject to the cited directive and must be disposed
of accordingly within Europe.
For more information, please visit www.kavo.com or contact your specialised
dental dealers.
For final disposal:
In Germany
To return an electrical device, you need to proceed as follows:
1. On the homepage www.enretec.de of enretec GmbH, you can download a
form for a disposal order under the menu item, eom. Download the disposal
order or complete it as an online order.
2. Enter the corresponding information to complete the order, and submit it as
an online order or by fax +49 (0)3304 3919 590 to enretec GmbH.
The following contact options are also available for questions and for initiat-
ing a disposal order:
Phone: +49 (0) 3304 3919-500
Email: eom@enretec.de and
Postal address: enretec GmbH, Geschäftsbereich eomRECYCLING®
Kanalstraße 17
D-16727 Velten
3. A unit that is not permanently installed will be picked up at the office.
A permanently installed unit will be picked up at the curb at your address on
the agreed date.
The owner or user of the device will have to bear the cost of disassembly,
transportation and packaging.
International
For country-specific information on disposal, contact your specialised dealers.
13 / 110

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.1 Purpose – Proper use
14 / 110
3 Product description
3.1 Purpose – Proper use
The dental operating lights KaVoLUX 540 LED U and T are designed for the illu-
mination of the oral cavity of a patient during the dental treatment. The lights
are mounted to the dental treatment units, the use is restricted to medical spe-
cialist staff in rooms used for medical purposes.
The type 540 LED T treatment light can also be mounted on the ceiling with an
adapter independent of a treatment unit.
The operation of the treatment light depends on the type of light used and is
accomplished via:
▪ Type 540 LED U: control panel of an intended treatment unit and move-
ment sensor of the light
▪ Type 540 LED T: keypad on the light head and movement sensor of the
light
The product complies with the requirements of DIN EN ISO 9680.
The KaVoLUX 540 LED U can be mounted to the following treatment units:
▪ ESTETICA E80 / E80 Vision
▪ ESTETICA E70 / E70 Vision
▪ ESTETICA E50 / E50 Life
The KaVoLUX 540 LED T can be mounted in the following positions/to the fol-
lowing treatment centres:
▪ on the ceiling
▪ Primus 1058 / 1058 Life
▪ Status 1080
▪ Globus 1078
▪ ESTETICA Sensus 1066
▪ ESTETICA Comfort 1065
▪ ESTETICA Standard 1063
▪ ESTETICA E30
▪ DSEclinical
▪ CENTRO support system
"Proper use" also includes compliance with all of the information in the operat-
ing instructions and ensuring that all inspection and servicing work is performed
as scheduled.
The overarching guidelines and/or national laws, national regulations and the
rules of technology applicable to medical devices for start-up and use of the
KaVo product for the intended purpose must be applied and followed.
KaVo accepts liability for the safety, reliability, and performance of components
supplied by KaVo, provided:
▪ installation, instructions, expansions, adjustments, changes or repairs were
carried out by technicians trained by KaVo or third parties authorised by
KaVo, or by the personnel of authorised distributors.

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.1 Purpose – Proper use
▪ the unit was operated in accordance with the instructions for use, care and
installation.
▪ the IT components supplied by the operator meet the technical require-
ments in the present instruction for use for hardware and software, and are
installed and set up according to the descriptions of these components.
▪ in the case of repairs, the requirements of IEC 62353 "Recurrent tests and
tests before start-up of electrical items of medical electrical equipment and
systems - general regulations" are met in full.
It is a responsibility of the user:
▪ to only use equipment that is operating correctly,
▪ to protect him or herself, the patient and third parties from hazards, and
▪ to prevent contamination from the product
The applicable national legal regulations must be observed during the use of the
device, in particular the following:
▪ Applicable regulations governing the connection and startup of medical
devices.
▪ Current occupational safety regulations.
▪ Current accident prevention regulations.
Regular servicing and safety checks are essential for the permanent assurance
of the operating and functional safety of the KaVo product and for the preven-
tion of damage and hazards.
Testing and maintenance intervals: Maintenance must be performed once a
year, the safety checks at intervals of 2 years. Shorter intervals for the safety
checks may be specified by the tester if necessary.
The following persons are authorised to conduct repairs and servicing and the
safety check on the KaVo product:
▪ Technicians of KaVo branch offices after appropriate product training.
▪ Specifically KaVo-trained technicians of KaVo franchised dealers.
In Germany, operators, equipment managers and users are obliged to operate
their equipment in accordance with the MPG regulations.
The services encompass all the test tasks required in accordance with § 6 of the
medical devices operator ordinance (Medizinprodukte-Betreiberverordnung, MP-
BetreibV).
Note
The product must be cleaned and serviced according to instructions if it is not
to be used for an extended period of time.
Note
The treatment light may no longer be operated if the functional safety and
the faultless condition of the device cannot be guaranteed.
15 / 110

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.2 Operating light KaVoLUX 540 LED U
16 / 110
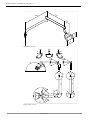
3.2 Operating light KaVoLUX 540 LED U
Note
The KaVoLUX 540 LED U operating light is only suitable for mounting to the
KaVo treatment centre ESTETICA E80, E70, E50 and E50 Life, as it can be
directly controlled via the treatment centre.
Components of the KaVoLUX 540 LED U:
①Mounting pole ②Swivel arm
③Spring arm ④3D joint
⑤Handles ⑥Mirror (optional)
⑦Operating light head

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.3 Operating light KaVoLUX 540 LED T
3.3 Operating light KaVoLUX 540 LED T
Note
The KaVoLUX 540 LED T operating light is designed exclusively for ceiling in-
stallation and mounting to the KaVo treatment centres
Primus 1058 / 1058 Life, Status 1080, Globus 1078, ESTETICA Sensus 1066,
ESTETICA Comfort 1065, ESTETICA Standard 1063, ESTETICA E30 as well as
for mounting to DSEclinical dental simulation units. The assembly kit (Mat.
no. 07243401) is required for installation.
Components of the KaVoLUX 540 LED U:
①Operating light head ②Handles
③3D joint ④Control panel
⑤Spring arm ⑥Swivel arm
⑦Ceiling mounting kit (Mat. no.
1.008.8406)
⑧Mirror (optional)
3.4 Dimensions and swing range
CAUTION
Collision with persons or pieces of equipment.
Collisions could be caused by the required degrees of freedom and the large
swivel range
▶Always move or swivel the operating light with great care.
17 / 110

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.4 Dimensions and swing range
18 / 110
843
659 120
240
300
Dimensions (in mm)
763
843
659
131°
131°
131°
131°
45°
320°
30°
30°
30°
30°
320°
118°
140°
140°
17°
17°
28°
45°
Swing ranges (in mm)

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.5 Rating plates
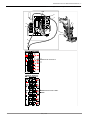
3.5 Rating plates
There is one rating plate for the treatment unit and, in the case of ceiling
mounting, one additional rating plate for the ceiling mounting kit,
The rating plate of the operating light is located on the top side of the swivel
arm.
Rating plate KaVoLUX 540 LED
①Device type ②Material number, depending on
type of device
③Year of manufacture - month -
serial number
④Supply voltage and power
⑤Reference number to product ⑥HIBC Code
⑦Type description ⑧VDE mark
⑨Disposal instructions (see intended
use)
⑩eLabeling ID
⑪CE mark ⑫Read and take note of the content
of accompanying documents
The rating plate of the ceiling mounting kit is fastened to the ceiling.
Rating plate KaVoLUX 540 LED T ceiling installation
①Device type ②Material number
③Year of manufacture - month -
serial number
④Supply voltage and power
⑤Reference number to product ⑥HIBC Code
⑦Disposal instructions (see intended
use)
⑧VDE mark
⑨eLabeling ID ⑩CE mark
⑪Read and take note of the content
of accompanying documents
19 / 110

Instructions for use KaVoLUX 540 LED U / T
3 Product description | 3.6 Technical data and requirements
20 / 110
3.6 Technical data and requirements
Photobiological safety (IEC 62471) Risk group 1 (low risk) The products
are safe under the majority of meth-
ods of application, except for the
case of prolonged exposition with
potentially direct eye exposure.
Electrical system for mounting the device
Input voltage 24 V AC
Frequency 50/60 Hertz
Power consumption max. 35 VA
Ceiling mounting and electrical system
Input voltage 100 – 240 V AC
Frequency 50/60 Hertz
Power consumption max. 50 VA
Electrical lead 3x1.5 mm2 in accordance with DIN
VDE 0100-710
Free end at assembly point 1000mm
Customer-provided fuse protection Automat C 16 or screw-plug fuse 10
A. Can be connected to the auto-
matic unit of the dental treatment
centre
Main fuse of the terminal T 500mA H / 250 V
Colour reproduction and illumination strength
Colour temperature normal light ca 4.000 to 6.000 Kelvin; preset
5.500 Kelvin (daylight quality)
Colour rendering index CRI >93 at 5,500 Kelvin Δc < 0.005
Normal light approx. 20,000 to 40,000 lux; preset
30,000 lux in accordance with
ISO 9680:2015
COMPOsave (compatible mode for
light-activatable restoration materi-
als)
approx. 8,000 to 15,000 Lux in ac-
cordance with ISO 9680:2015
Dimmed light approx. 8,000 to 15,000 Lux / ap-
prox. 4,000 K
Typical distribution of illumination in-
tensity
blue 75%, green 50%, yellow 10%
of the illumination intensity
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
Page is loading ...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
-
 85
85
-
 86
86
-
 87
87
-
 88
88
-
 89
89
-
 90
90
-
 91
91
-
 92
92
-
 93
93
-
 94
94
-
 95
95
-
 96
96
-
 97
97
-
 98
98
-
 99
99
-
 100
100
-
 101
101
-
 102
102
-
 103
103
-
 104
104
-
 105
105
-
 106
106
-
 107
107
-
 108
108
-
 109
109
-
 110
110
KaVo LUX 540 LED U Instructions For Use Manual
- Type
- Instructions For Use Manual
- This manual is also suitable for
Ask a question and I''ll find the answer in the document
Finding information in a document is now easier with AI
Related papers
-
KaVo ESTETICA E70/E80 Vision Operating instructions
-
KaVo KaVo Primus 1058 Life TM Instructions For Use Manual
-
KaVo INTRA LUX KL 703 LED Operating instructions
-
KaVo INTRA LUX KL702 Operating instructions
-
KaVo ERGOcom 2 Operating instructions
-
KaVo ESTETICA E50 Care Instructions
-
KaVo ELECTROmatic - Plus Operating instructions
-
KaVo ERGOcom 3 User Instructions
-
KaVo QUATTROcare PLUS 2124 A Instructions For Use Manual
-
KaVo SMARTair plus/SMARTair plus mobile Operating instructions
Other documents
-
Pulse LCD-PM200 Operating instructions
-
MG ML0YIS1A User manual
-
 G-TIDE E70 User manual
G-TIDE E70 User manual
-
 MIMSAL MIMLED 600 User manual
MIMSAL MIMLED 600 User manual
-
 imoshion Ring Led Light User manual
imoshion Ring Led Light User manual
-
Belmont Clesta LED AL301, 302, 305 Installation guide
-
ASI Triton Advanced Dental System 2025M/AR Operation & Service Manual
-
 Coremorrow Modular E70 Series Piezo Controller User manual
Coremorrow Modular E70 Series Piezo Controller User manual
-
NSK Ti-Max X500 Operating instructions
-
NSK PER-BA-P Operating instructions

















































































































