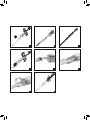
Stryker DirectInject is a sterile, self-setting calcium phosphate cement system designed to repair neurosurgical burr holes, contiguous craniotomy cuts, and other cranial defects. It can also be used for augmentation or restoration of bony contour in the craniofacial skeleton, including the cranial and zygomatic bones. The cement is moldable upon injection and hardens under normal body conditions to form hydroxyapatite. DirectInject is provided pre-filled in a double-barrel Delivery Syringe system and includes two Mixer-Cannulae and a Spatula for easy application.
Stryker DirectInject is a sterile, self-setting calcium phosphate cement system designed to repair neurosurgical burr holes, contiguous craniotomy cuts, and other cranial defects. It can also be used for augmentation or restoration of bony contour in the craniofacial skeleton, including the cranial and zygomatic bones. The cement is moldable upon injection and hardens under normal body conditions to form hydroxyapatite. DirectInject is provided pre-filled in a double-barrel Delivery Syringe system and includes two Mixer-Cannulae and a Spatula for easy application.










-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
Stryker DirectInject is a sterile, self-setting calcium phosphate cement system designed to repair neurosurgical burr holes, contiguous craniotomy cuts, and other cranial defects. It can also be used for augmentation or restoration of bony contour in the craniofacial skeleton, including the cranial and zygomatic bones. The cement is moldable upon injection and hardens under normal body conditions to form hydroxyapatite. DirectInject is provided pre-filled in a double-barrel Delivery Syringe system and includes two Mixer-Cannulae and a Spatula for easy application.
Ask a question and I''ll find the answer in the document
Finding information in a document is now easier with AI
Related papers
-
Stryker Xia 4.5 User manual
-
Stryker System 9 Sterile Battery Charger Operating instructions
-
Stryker 5100-015-250 User manual
-
Stryker CrossFlow User manual
-
Stryker Reprocessed MyoSure LITE Tissue Removal Device User manual
-
Stryker SPR Plus User manual
-
Stryker Crossfire 2 User manual
-
Stryker 1488 User manual
-
Stryker LF1923 User manual
-
Stryker 6290-100-000 Operating instructions
Other documents
-
jbc SB03CC User manual
-
 Merit Medical STABILIT Operating instructions
Merit Medical STABILIT Operating instructions
-
 Merit Medical Stabilit Bone Cement and Saturate Mixing System Operating instructions
Merit Medical Stabilit Bone Cement and Saturate Mixing System Operating instructions
-
 Merit Medical Arcadia Steerable Balloon and Straight Balloon Catheter Operating instructions
Merit Medical Arcadia Steerable Balloon and Straight Balloon Catheter Operating instructions
-
jbc SB05CC User manual
-
jbc DPM-B DPM User manual
-
 Hologic Tumark Vision Operating instructions
Hologic Tumark Vision Operating instructions
-
Abbott CentriMag Reference guide
-
Bard DUET Operating instructions
-
Aesculap Univation X Surgical Technique













