The participant in the testing program should enter the test
results into the NAVICA™ website. The record will remain
in NAVICA™ for reference.
• Follow the instructions on the NAVICA™ website. For
more detailed instructions on entering your test result,
here is a Quick guide.
• Your results will automatically be shared with your
organization. No action is needed from you as long as
you stay connected.
• Test results will be saved in NAVICA™, so you can print
your results to share with other organizations as needed.
Get your tests Test Record results
NAVICA™ Q&A
The BinaxNOW™ COVID-19 Antigen Self Test has not been FDA cleared
or approved. It has been authorized by the FDA under an emergency use
authorization. It has been authorized only for the detection of proteins from
SARS-CoV-2, not for any other viruses or pathogens, and is only authorized
for the duration of the declaration that circumstances exist justifying the
authorization of emergency use of in vitro diagnostics for detection and/or
diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food,
Drug and Cosmetic Act, 21 U.S.C. § 360bbb 3(b)(1), unless the declaration
is terminated or authorization is revoked sooner. For serial testing, the
BinaxNOW COVID-19 Antigen Self Test should be performed twice
over 3 days, at least 24 hours (and no more than 48 hours) apart. For
symptomatic use, a single test can be used.
© 2022 Abbott. All rights reserved. All trademarks referenced are trademarks
of either the Abbott group of companies or their respective owners. Any
photos displayed are for illustrative purposes only. COL-07087-02 01/22
To learn more about antigen testing and the science
behind it, visit BinaxNOW-selftest.abbott
Participants will collect their own swab specimens at home via
an easy-to-use, painless nasal swab that takes less than
1 minute.
• Review BinaxNOW™ COVID-19 Antigen Self Test Video
and instructions provided in the test kit to learn about the
test and to familiarize yourself with the test materials and
procedure prior to performing any tests.
• Complete the test by following the instructions in the
test kit.
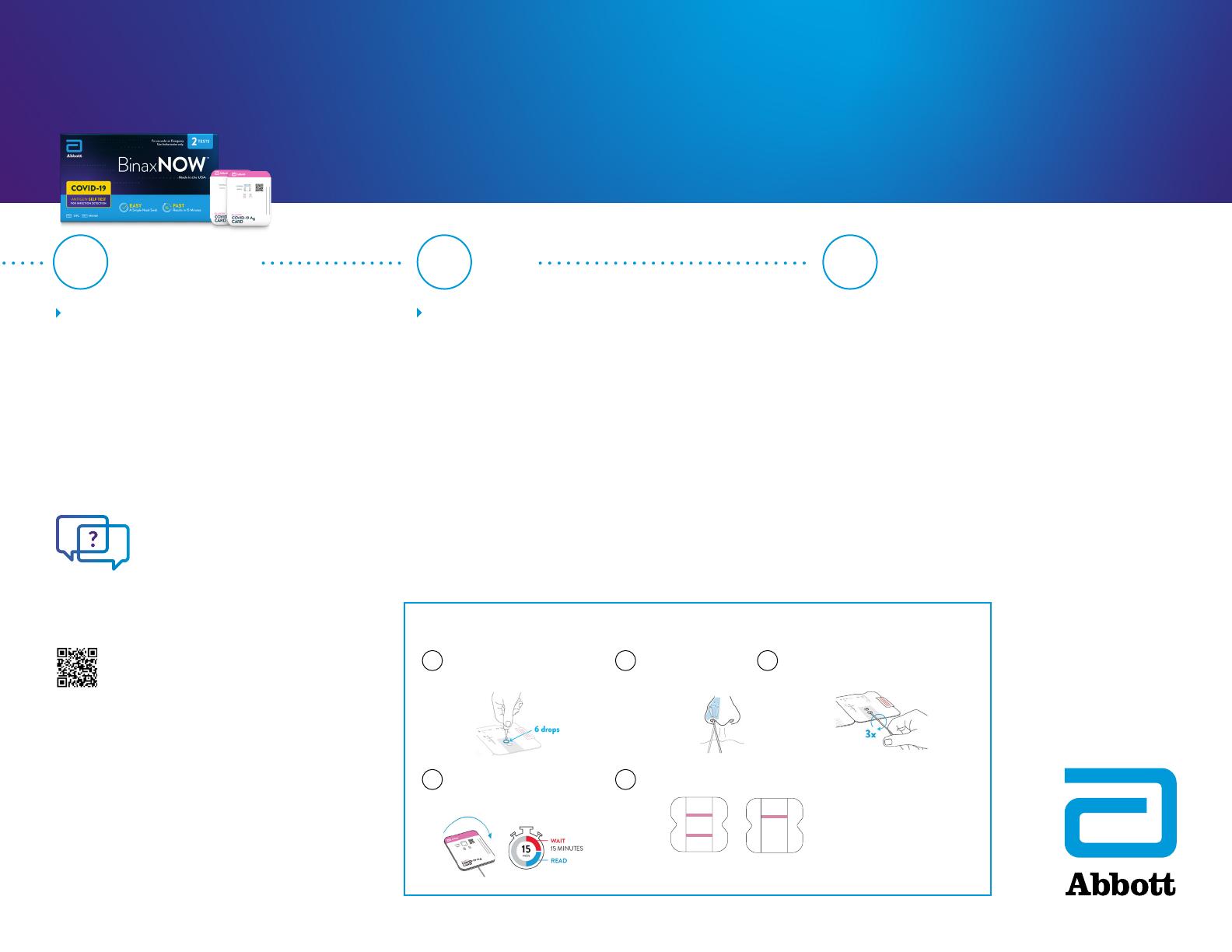
TEST PROCEDURE
Add the extraction reagent
1 2 Swab both nostrils
4Close the test card; wait
15 minutes
3Insert sample swab into the test card
and rotate the swab shaft three times
5Results are read visually
NEGATIVE
RESULT
POSITIVE
RESULT
PINK/PURPLE
CONTROL LINE
PINK/PURPLE
SAMPLE LINE
AT 15–30 MINUTES
654
BinaxNOW™• Antigen testing is designed to identify the proteins
of the SARS-CoV-2 virus
• The BinaxNOW™ COVID-19 Antigen Test
detects active COVID-19 infection and can be
used for people with and without symptoms
• This test is packaged with two tests per box so you
can test yourself twice over 3 days, at least 24
hours (and no more than 48 hours) apart
• By testing more frequently, known as serial testing,
you may detect COVID-19 more quickly and
reduce the spread of the infection
IS AN ANTIGEN TEST
This area is editable for you to describe how the test kits will be distributed.
Examples:
• Please pick up your kits at the front desk in the lobby on Monday, 8/15, between
1 and 3 p.m.
• Test kits will be sent directly to your home from Abbott.
Add your testing program guideline for testing frequency.
Example:
• You will be sent a reminder to test on Sunday and Tuesday of each week.
• Testing verification is required before attending your organization on Monday and Thursday mornings.








